ReelVision
Your fastest, quality-assured route to market
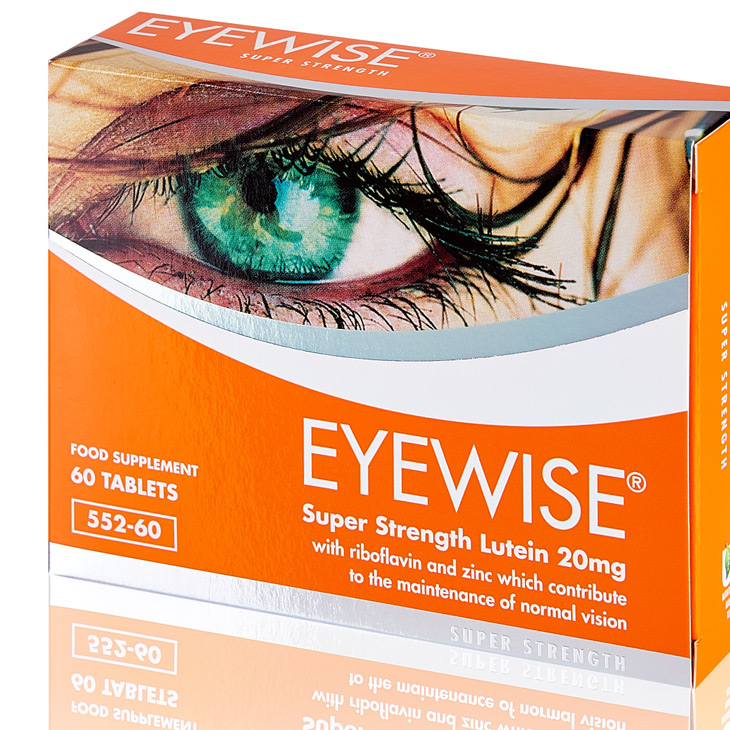
Security guarantee, reliability, consistency and premium quality are all key factors for the pharmaceutical industry.
We have positioned our pharmaceutical packaging business both technically and commercially in order to best serve these demands and produce small order batches of printed cartons which eliminates the risk of redundant stock.
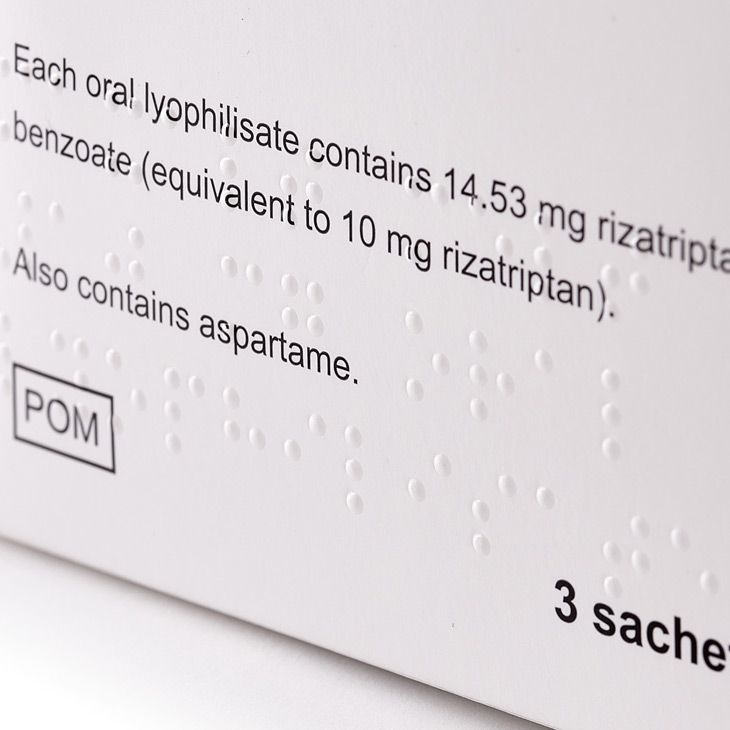
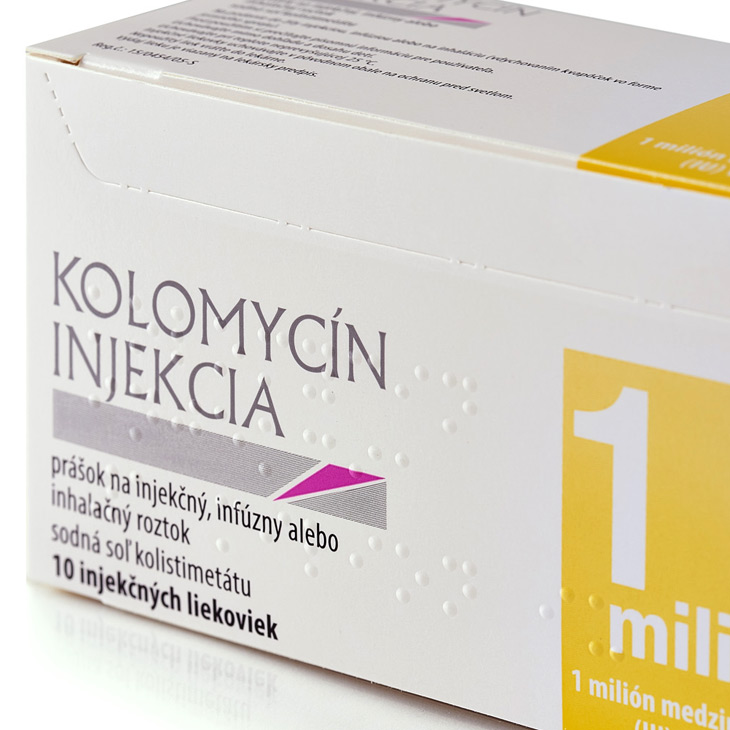
Reelvision Print has a company policy of non-composite printing and will never manufacture more than one design on a set of printing plates. This production commitment combined with the one-pass printing, die-cutting and Braille embossing process guarantees that a product admixture will never occur.
Colour consistency, premium graphics, flexible service and creativity are some of the major requirements of the Cosmetics, Health and Personal Care market.
Our unique production process ensures we are equipped to deliver these critical needs combined with low volume production which reduces stock levels and increases cash flow.
Manufacturing from a web of board creates the opportunity for Reelvision Print to add value to the material in terms of holographic security tags and foil or film substrates. This unique print process will naturally deliver high lustre metallic inks and brilliant gloss UV varnishes. Our ability to perfect in-line means we can offer our customers printing on the back and front of the board in one pass of the machine, utilising the eight available print stations.
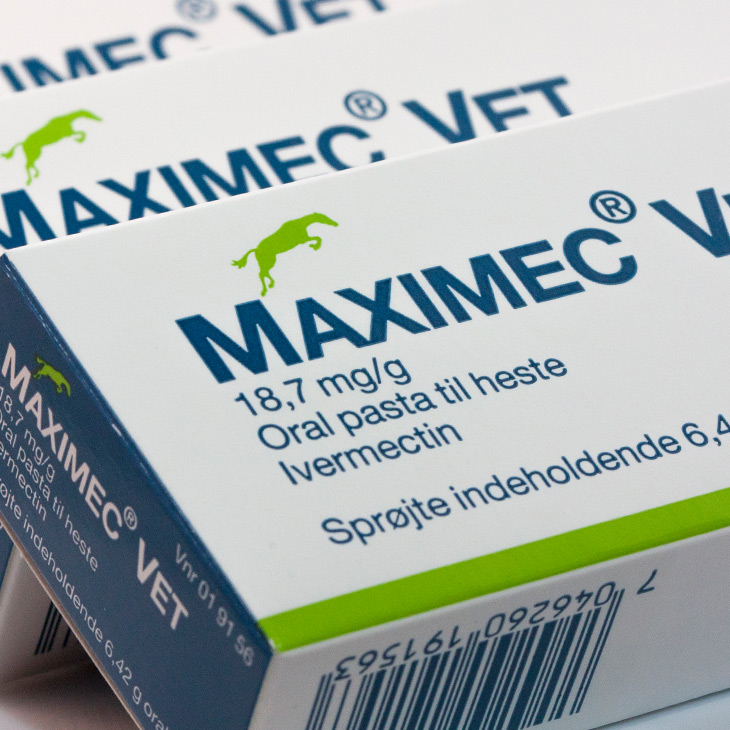
Product security, reliability, short lead-times and guaranteed service are all requirements for this market.Our technological advancement in manufacturing techniques means we can deal with these needs and supply small order quantities, enabling flexibility for design changes without the need to write-off stock.
Pioneering new production techniques has been the foundation for Reelvision Print's success in attracting new business and our management team has developed long-term trading relationships. Customers expect the best and Reelvision's unique approach ensures this high level of service is maintained and challenged in order to continuously look for process improvements.